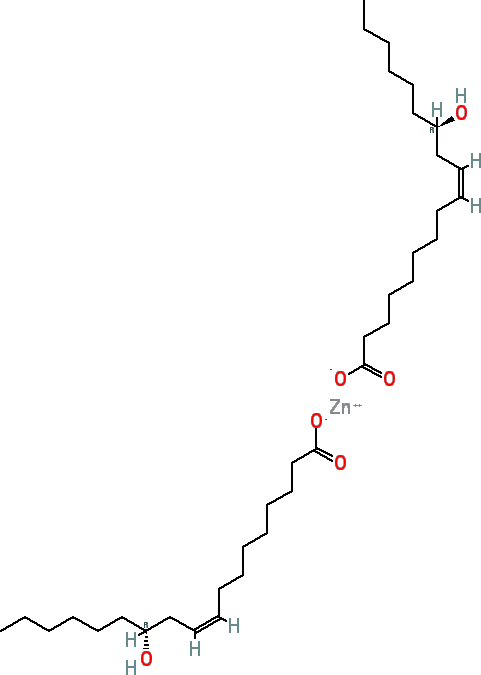
Zinc Ricinoleate
Safety Information
U.S. Food and Drug Administration
The U.S. Food and Drug Administration (FDA) includes castor oil on its list of natural flavoring substance and on its list of multipurpose direct food additives. Castor oil is also classified by the FDA as safe and effective as a stimulant laxative.
Expert Panel for Cosmetic Ingredient Safety
The safety of Ricinus Communis (castor) seed oil, cetyl rinoleate, ethyl ricinoleate, glyceryl ricinoleate, glyceryl ricinoleate SE, glycol ricinoleate, hydrogenated castor oil, isopropyl ricinoleate, methyl ricinoleate, octyldodecyl ricinoleate, potassium ricinoleate, ricinoleic acid, sodium ricinoleate and zinc ricinoleatehave been assessed by the Expert Panel for Cosmetic Ingredient Safety. The Expert Panel evaluated the scientific data and noted the overall pattern of use of these ingredients in different product categories. The Expert Panel concluded that castor oil and its derivatives were safe for use as cosmetic ingredients.
The Expert Panel considered that the available data on Ricinus Communis (castor) seed oil, hydrogenated castor oil, ricinoleic acid, and salts and esters of ricinoleic acid were sufficient for evaluating the safety of these ingredients. Because Ricinus Communis (castor) seed oil contains ricinoleic acid as the primary fatty acid group, safety test data on the oil was considered broadly applicable to this entire group of cosmetic ingredients. Overall, the available data demonstrated few toxic effects in acute, subchronic or chronic toxicity tests. Additionally, there were no genotoxic effects of castor oil in in vitro or in vivo tests. UV absorption spectra on Ricinus Communis (castor) seed oil and glyceryl ricinoleate indicated maximum absorbance at 270 nm, suggesting there would be no photosensitization potential of glyceryl ricinoleate or Ricinus Communis (castor) seed oil in human subjects exposed to the sun. Reactions classified as either significantly irritating or allergic were not observed in studies on ethyl ricinoleate, and the Expert Panel concluded that the castor oil derivatives were not sensitizers. The Expert Panel also determined that these ingredients may be used safely in aerosolized products because packaging and use ensure that particulates are not respirable.
Link to FDA Code of Federal Regulations regulation for castor oil:
Ricinus Communis (castor) seed oil, cetyl rinoleate, ethyl ricinoleate, glyceryl ricinoleate, glyceryl ricinoleate SE, glycol ricinoleate, hydrogenated castor oil, isopropyl ricinoleate, methyl ricinoleate, octyldodecyl ricinoleate, potassium ricinoleate, ricinoleic acid, sodium ricinoleate and zinc ricinoleate may be used in cosmetics and personal care products marketed in Europe according to the general provisions of the Cosmetics Regulation of the European Union.
Link to the EU Cosmetic Regulation:
http://europa.eu/legislation_summaries/consumers/product_labelling_and_p…
The Joint FAO/WHO Expert Committee on Food Additives has established an acceptable daily intake of 0-0.7 mg castor oil/kg body weight.
https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-120.pdf