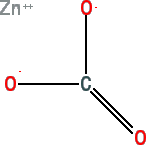
Zinc Carbonate
Safety Information
U.S. Food and Drug Administration (FDA)
The U.S. Food and Drug Administration (FDA) includes calcium carbonate, magnesium carbonate and potassium carbonate on its list of direct food substances affirmed as Generally Recognized as Safe (GRAS). Calcium carbonate and magnesium carbonate are FDA approved as active ingredients in over-the-counter (OTC) antacid drug products. Zinc carbonate is approved for use in OTC skin protectant drug products at concentrations of 0.2 to 2%. Calcium carbonate is an FDA-approved color additive for use in drugs.
Link to FDA Code of Federal Regulations for calcium carbonate
Link to FDA Code of Federal Regulations for magnesium carbonate
Link to FDA Code of Federal Regulations for potassium carbonate
Link to FDA Code of Federal Regulations for zinc carbonate
Expert Panel for Cosmetic Ingredient Safety
The Expert Panel for Cosmetic Ingredient Safety has deferred evaluation of these ingredients because the safety has been assessed by FDA. This deferral of review is according to the provisions of the Expert Panel procedures.
Calcium carbonate, magnesium carbonate, potassium carbonate and zinc carbonate may be used in cosmetics and personal care products marketed in Europe according to the general provisions of the Cosmetics Regulation of the European Union.