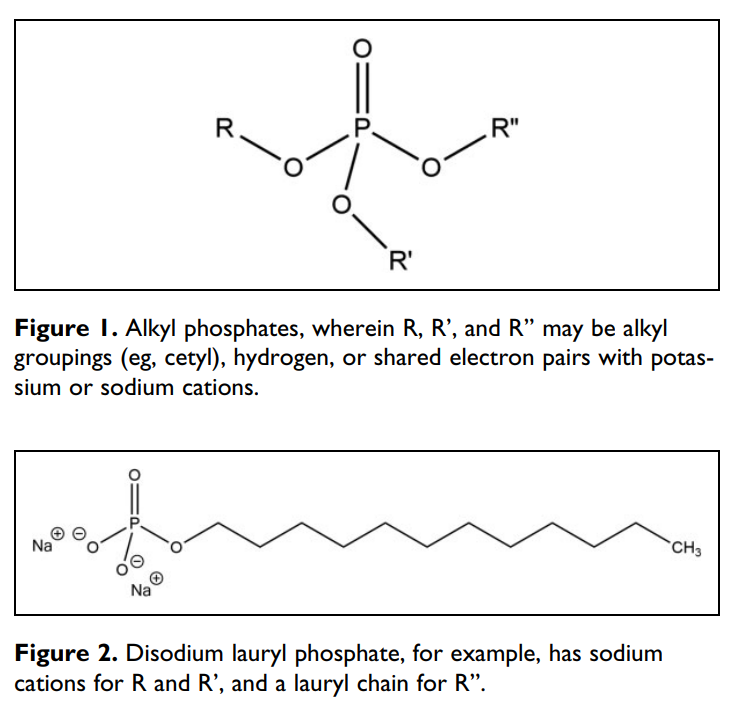
Alkyl Phosphates
Safety Information
Expert Panel for Cosmetic Ingredient Safety
The Expert Panel for Cosmetic Ingredient Safety (formerly the Cosmetic Ingredient Review Expert Panel) evaluated available safety information for 28 alkyl phosphate ingredients and concluded they are safe in the present practices of use and concentration in cosmetics when formulated to be non-irritating to skin and eyes:
| Potassium Cetyl Phosphate | Lauryl Phosphate |
| Potassium C9-15 Alkyl Phosphate | Myristyl Phosphate* |
| Potassium C11-15 Alkyl Phosphate* | Octyldecyl Phosphate* |
| Potassium C12-13 Alkyl Phosphate | Oleyl Ethyl Phosphate* |
| Potassium C12-14 Alkyl Phosphate* | Oleyl Phosphate* |
| Potassium Lauryl Phosphate | Sodium Lauryl Phosphate* |
| C8-10 Alkyl Ethyl Phosphate* | Stearyl Phosphate |
| C9-15 Alkyl Phosphate | Dicetyl Phosphate |
| C20-22 Alkyl Phosphate | Dimyristyl Phosphate* |
| Castor Oil Phosphate | Dioleyl Phosphate |
| Cetearyl Phosphate* | Tricetyl Phosphate* |
| Cetyl Phosphate | Trilauryl Phosphate* |
| Disodium Lauryl Phosphate* | Trioleyl Phosphate |
| Disodium Oleyl Phosphate* | Tristearyl Phosphate* |
*Not reported to be in current use. Were these ingredients to be used in the future, the expectation is that they would be used in product categories and at concentrations comparable to others in this group.
Much of the data included in the Expert Panel’s safety assessment were found on the European Chemicals Agency (ECHA) website. The Expert Panel also relied on data demonstrating the safety of several structurally similar ingredients containing chain-length distributions that overlap with alkyl phosphates.
Finally, the Expert Panel was concerned that the potential exists for ocular and/or dermal irritation with the use of products formulated using alkyl phosphates and they specified that products containing them must be formulated to be non-irritating to skin and eyes.
U.S. Food and Drug Administration (FDA)
Potassium lauryl phosphate can be used as an optional finish component in poly(phenyleneterephthalamide) resins, which are indirect food additives intended for repeated contact with food. In this application, the total weight of potassium lauryl phosphate is not to exceed 1% of the base polymer [21CFR177.1632]. Tristearyl phosphate is approved as an indirect food additive as a substance permitted to be used in the formulation of defoaming agents used in the manufacture of paper and paperboard [21CFR176.210].
All of the alkyl phosphates reviewed by the Expert Panel are marketed in Europe according to the general provisions of the Cosmetics Regulation of the European Union and used as surfactants, emulsifiers or plasticizers in cosmetics.